Peerless Tips About How To Clean Up Nitric Acid

It can be utilised more successfully at lower temperatures than caustic solutions, requiring less heating.
How to clean up nitric acid. Develop a standard operating protocol (sop), and include details for waste. The entire spill kit containing the clean up materials can then be labeled and. Safe use practices minimize/eliminate use and storage of nitric acid whenever possible.
Affected skin will require flushing with soap and lukewarm water for at least 30 minutes. Process nitric acid is used for cleaning glassware and creating aqua regia for selenium analysis. I understand the corrosive dangers of nitric acid, and most of the important dangers of its reactivity (e.g.
To safely dispose of waste nitric acid: Fill the drip system with a solution of 38% nitric acid (3 litres of 38% nitric acid to 100. Neutralize acids with soda ash or sodium.
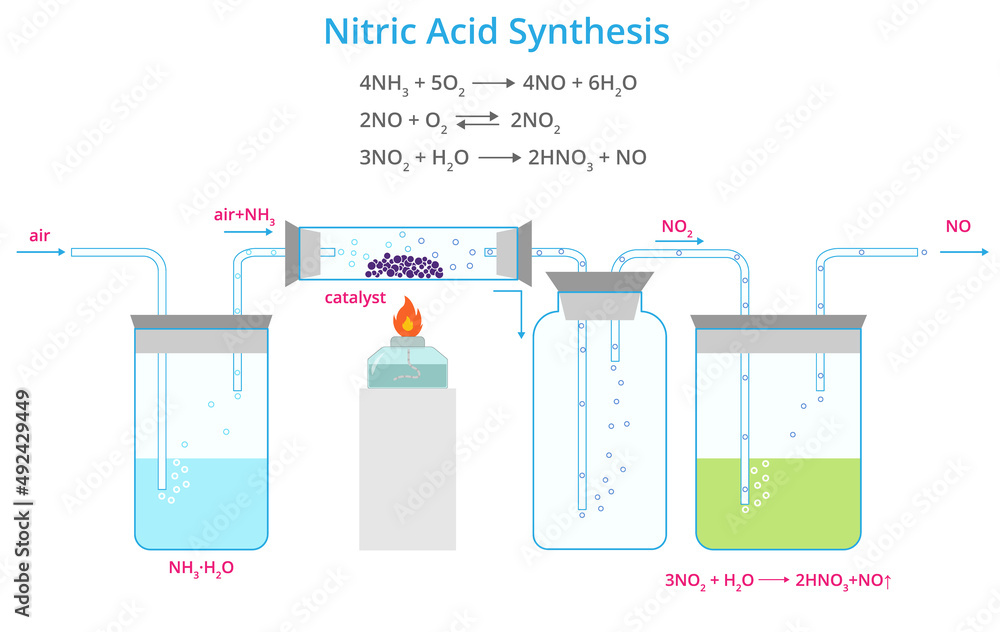
It is produced by a high temperature oxidation of ammonia with oxygen catalyzed by a. It is a highly corrosive mineral acid. Small or dilute concentrations (10 ml of >50% or 100 ml of dilute) can be cleaned up using a spill kit.
Absorbent pads are highly effective at cleaning up a wide variety of chemicals, including acids. Never allow chlorine bleach to come in contact with nitric acid. Throw away food and packaging materials within a 25 foot circle of vomit.
Nitric acid nitric acid is the inorganic compound with the formula h n o 3. Octaneenergydrink on february 13, 2024: Strong acids and bases can be very.
What makes nitric acid an excellent cleaning chemical? Never mix inorganic acids and organic solvents. Do not store nitric acid near materials it might react.
Make sure you cover all areas of your skin to protect against burns. Dispose of soiled items/waste/gloves in a plastic trash bag. It’s the one used to make bombs (tnt) and fertilizers.
5:00 neutralizing an acidic or caustic material during spill cleanup makes the material safer to handle and dramatically reduces disposal costs. You simply place them directly in the spill and they’ll. However, be careful because the neutralization process is often vigorous, causing splashes and yielding large amounts of heat.
As previously mentioned, both the fumes and direct exposure to liquid nitric oxide are hazardous to humans. Nitric acid is one of the most common mineral acids in the chemical industry. Enhance your training performance by drinking the #1 clean hydrating energy drink on the market,.